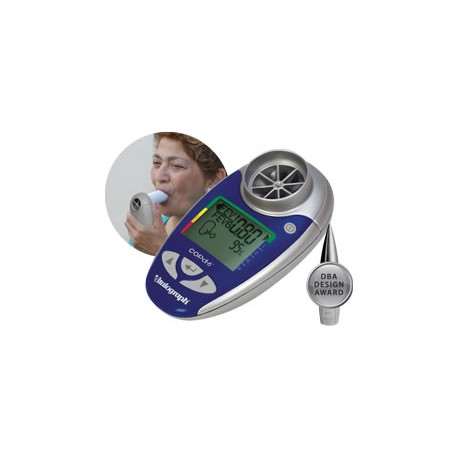
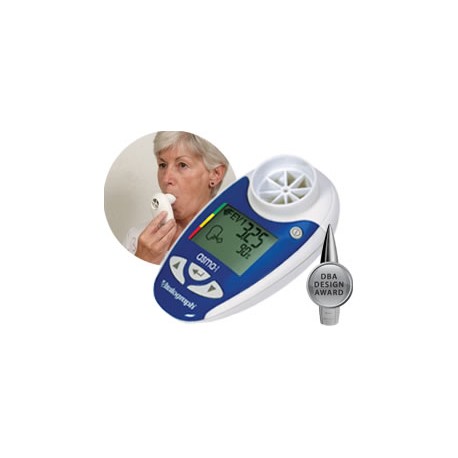
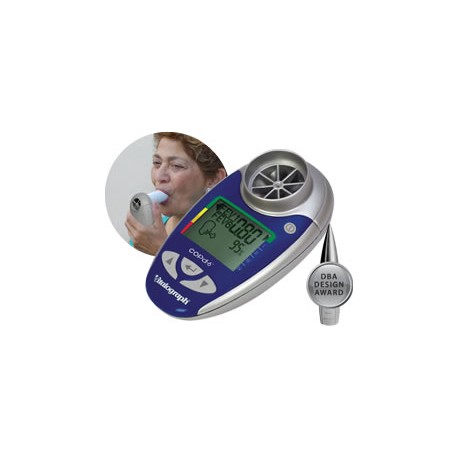
Vitalograph - copd-6 USB Respiratory Screener
Product Properties The Vitalograph copd-6™ BT identifies those at risk of COPD at the pre-symptomatic stage, screening out those whose FEV1 is normal. The screener allows early medical intervention and facilitates better clinical outcomes. Generates reports via USB connection and Vitalograph Reports software. Monitors COPD patients using their obstructive index – FEV1 as a percent of predicted Displays FEV1, FEV6, ratio, COPD classification and lung age Automatically sends data after each blow Can also send session data Large, easy-to-read display One of our 4000 Series of respiratory monitors and screeners, the COPD-6 USB comes with: Pouch Instructions for Use USB Cable Vitalograph Reports Software 2 x AAA Batteries Get the tools to build your own application on Windows®, Apple iOS and Android™ platforms to integrate with the asma-1 USB using our comprehensive API software developers’ kit (sold separately). The SDK provides simple and fast integration for Telehealth providers, enabling third-party applications to interface with Vitalograph devices. Designed for providers of home hub and telemedicine solutions, the SDK includes license agreement, software code, detailed device API and access to the R&D Technical Support Hotline. Providing: Access to all test data including measured parameters, test quality and zone information stored on the device Ability to set and control personal best FEV1, zone settings, date and time Ability to integrate with the Series 4000 respiratory monitors and screeners (USB, BT models) Key Features Facilitates ‘case selection’ so that spirometry can be focused on those likely to be diagnosed with COPD. Screens out anyone with normal FEV1 without the risk of false COPD negatives. Monitors COPD patients using their obstructive index which is FEV1 as a percent of predicted. Displays FEV1, FEV6, ratio and % predicted, obstructive index, COPD classification and lung age. Built-in quality of blow indicator. Displays the GOLD COPD classification (stage I – IV) to help identify the need for a change in the patient’s management plan. Requires only minimal instruction for use by non-respiratory specialists. Suitable for use with Bacterial Viral Filters (BVF™) providing 99.999% cross-contamination efficiency. API software developers’ kit is required for integration.